Thanks to Gary Cornell and Devin Thomas for becoming Knife Steel Nerds supporters on Patreon.
Last Time, in Steel History….
When we last left steel history, the first high speed steels had been developed which had led to an explosion in steel development. I covered all of this in The History of the First Tool Steel. A few highlights of that article:
- Steels can harden in air rather than with quenching in oil or water if sufficient manganese, chromium, or other elements are added.
- When high tungsten steels are hardened with very high temperatures they show high “red hardness” meaning they can be operated at relatively high temperatures without losing their hardness. This led to the ability to use cutting steels at much higher speeds, i.e. “High speed steel.”
- A small vanadium addition (~1%) led to improved cutting behavior.
A tungsten-alloyed high speed steel later named T1 became the dominant high speed steel for several years to follow, and in fact is still used today:
![]()
Replacement of Molybdenum
It was also discovered relatively early that molybdenum-alloyed steels could have to similar behavior to tungsten-alloyed steels because both elements form the very small carbides during high temperature tempering that leads to their red hardness [1]. Also, because molybdenum is approximately half the atomic weight of tungsten, tungsten can be replaced by only half of the amount of molybdenum. However, the proliferation of molybdenum-alloyed high speed steels was initially slow. James P. Gill, in his master’s thesis published in 1922 [2], stated that the primary reason that molybdenum was not used in high speed steel was because of the loss of molybdenum during high temperature forging and heat treatment through “molybdenum oxide volatilizing from the steel.” He was also not convinced that the use of molybdenum would yield properties that could match the tungsten-alloyed high speed steels [2]: “During the last few years the foreign countries especially England have made startling statements for the use of molybdenum in high speed steel when most of these steels that they claimed so splendid were proven failures a few years ago in America when compared to our best high speed steels.”

Molybdenum Oxide [3]
James P. Gill – Pioneering Steel Metallurgist
James Gill attended Missouri School of Mines and Metallurgy, later named University of Missouri-Rolla and now Missouri University of Science and Technology, where he completed a bachelor’s degree in Mining Engineering and Metallurgy and a master’s degree in Metallurgy. When graduating with his bachelor’s degree he was described as follows [4]:
It is a “toss up” as to whether “Jimmie” is a student or a faculty member this year. Takes delight in worrying the Juniors about the four things a good fusion should do. He has the reputation of being the most extensive second-hand dealer in Rolla and the most consistent and impartial “fusser” in school.

James P. Gill at the time of graduation with his bachelor’s degree [4]
He left school to work for the Vanadium Alloys Steel Company (VASCO) in Latrobe, PA, and remained with the company the rest of his life [1]. He made numerous contributions to steel including many patents and articles. In 1936 he published a book of 136 pages called Tool Steels which was a series of five lectures presented in 1934 to the American Society for Metals (ASM). This book is generally considered the first about modern tool steel. The book is an incredible summary of the knowledge of steel at that time, and is readable even today with surprisingly few errors that would be known with all of the metallurgy knowledge that has been gained since. In contrast, in the book “Tool Steel Simplified” published in 1937, Frank R. Palmer dedicated an entire chapter to a property called “Timbre” which is an outdated, dubious term that is essentially a catch-all for any steel property that they could not describe with then-common analysis methods.
This book, Tool Steels, is a testament to Gill’s commitment to sharing metallurgy knowledge and his proficiency in writing and teaching. He also greatly expanded the book in a second edition published in 1944 along with co-workers. After Gill’s death other VASCO metallurgists updated the book with a 3rd edition (1962) and 4th edition (1980). Reading the different editions of Tool Steels is fascinating because you can track the evolution of tool steel knowledge through the years.
Molybdenum Continued
Gill still remained unconvinced that molybdenum alloyed high speed steels were the future in his 1936 Tool Steels, saying, “All reports from these experiments and trials were favorable, some so much as to excite suspicion that some of the claims might be attributed to over-enthusiasm.” He did state, however, that molybdenum could be used to replace tungsten, “should tungsten be unavailable due to war, etc.” which was rather prescient. He did not believe that molybdenum-alloyed steels would take over because it didn’t have “any characteristics that make it superior to [tungsten-alloyed steel] other than cost.” And he repeated that high temperature operations would lead to loss of molybdenum to oxidation, making it difficult to work with. By the 1944 edition of Tool Steels, however, he reported that the loss of molybdenum due to oxidation was found to be untrue, and that the issue instead was due to the acceleration of decarburization (loss of carbon) at high temperature, and therefore required atmospheric protection of some kind to maintain hardness at the surface [1]. While additions of molybdenum rather than tungsten gave similar hardness at high tempering temperatures, molybdenum-alloyed steels were not found to maintain red hardness as well in operation, leading to the development of combined additions of both molybdenum and tungsten [1]. Despite these issues, molybdenum-alloyed, or combined molybdenum- and tungsten-alloyed high speed steels became the dominant alloys in the USA by the 50’s or 60’s, and this was due to several factors:
- There were significant tungsten shortages during World War II and the Korean War because the majority of tungsten is imported [1].
- Salt bath heat treating of high speed steels became much more common which offered protection against decarburization [1].
- The realization that molybdenum-alloying led to a lower melting point, meaning the optimum processing temperatures are lower. Too high austenitization led to poor performance in earlier experimentation [1].
- The operation of Climax mine by the Climax Molybdenum company, for increased availability of molybdenum [5].
High Vanadium Steels
Despite Gill’s skepticism about replacing tungsten with molybdenum, he patented several steels with high molybdenum content. However, the purpose of the patents was not to patent molybdenum alloying but instead to patent higher vanadium steels. He did work for the Vanadium Alloys Steel Company, after all. The issue with adding higher additions of vanadium was that beyond a certain point the hardness of the steel would drop significantly [6]:

The higher vanadium content would lead to a loss of carbon in solution in austenite, and also lead to ferrite present even at high temperature, as vanadium is a ferrite “stabilizer.” If the steel does not fully transform to austenite then it cannot transform to the hard martensite phase and instead has relatively soft ferrite; I covered this in part in Austenitizing Part 1 and What Makes Quenched Steel so Hard? This effect of vanadium is confirmed with thermodynamic calculations using JMatPro for a similar composition, where the replacement of austenite with ferrite can be seen:

Kinzel and Burgess found that for every 1% increase of vanadium beyond the standard T1 composition an extra 0.2% carbon was necessary so the steel could be fully austenitized and therefore achieve full hardness after quenching. They found a “preferred composition” of 1.5% carbon and 5% vanadium which is approximately the same as T15 high speed steel which was developed over a decade later. Surprisingly, the effect of vanadium at that time was not known; Kinzel and Burgess proposed that perhaps it was due to 1) grain refinement or 2) enhanced solubility at high temperature so that precipitation strengthening is enhanced. The high hardness of the vanadium carbide contributing to wear resistance was not mentioned.
Despite these significant findings of Kinzel and Burgess, no high vanadium high speed steels were commercialized, or at least did not achieve any significant popularity. It is not clear if Gill was aware of their research at the time, but in 1937 he submitted a patent application reporting the finding that carbon content needs to be significantly higher when greater amounts of vanadium are added [7][8]. In part this is because with the hard vanadium carbides that are formed, carbon is necessary to form the carbides while also having carbon “left over” for being present in austenite and finally martensite for hardness. The carbon also stabilizes austenite at high temperature, so that the ferrite problem is not present, as is shown in this JMatPro simulation with increasing carbon content for a 4% vanadium steel:

Gill studied steel with carbon contents as high as 3.0% when sufficient vanadium was added. Such high carbon contents were previously thought to lead to unworkable steel, but Gill found that the steels could be readily forged [8]. He also found that these steels had very high hardness and wear resistance but could still be annealed to low hardness for machining [8]. The good properties, in part, are because vanadium carbides are extremely hard and therefore lead to great improvements in wear resistance, as can be seen on this chart showing carbide hardness, where it is shown that vanadium carbide is more than twice as hard as iron carbides (Fe), chromium carbides (Cr), or W/Mo high speed steel carbides [9]:

James Gill, M4 Steel, and Legacy
This discovery of high carbon and high vanadium steels led to the development of M4 high speed steel by Gill [10], and later to M15 and T15 with higher vanadium content for superior wear resistance:

The original patent for M4 [7] was for a Molybdenum containing steel only (8-9% Mo), but shortly after it was modified for both Mo and W [11]. This modification was likely because of the building popularity of the Mo-W M2 steel at the same time, and also because a reduction in Mo down to 5-6% reduced the tendency to decarburization [11]. The even higher vanadium T15 appeared as “Vasco Supreme” by 1946 [12], and M15 as “Vasco Supreme A” by 1951 [13]. Those high speed steels are still used today, and M4 still sees use as a knife steel, particularly with the powder metallurgy version. The findings about combinations of higher carbon with high vanadium later led to the development of many high vanadium steels. There are many knife steels used today with relatively high vanadium content such as Vanadis 4, 10V, S30V, S90V, etc.
James P. Gill died in 1961 while still in the position of chairman of the board and president of VASCO. He had formerly been the president of the American Society for Metals from 1940-1941. His legacy was continued through publication of updated Tool Steels books by George A. Roberts and others who were employees of VASCO. Roberts’ last edition was in 1980, and an updated and abridged 5th edition was written by George Krauss of Colorado School of Mines and released in 1998. It doesn’t look like a 6th edition is in the cards at this point, but crazier things have happened. However, in the Preface to the 2016 book Tool Steels: Properties and Performance, Rafael Mesquita said that he attempted to write a book that is worthy of following Tool Steels, which again shows how influential the writings and research of James Gill continue to be.

Photo of James P. Gill [1]
Bonus! The First Tool Steels Book
As part of this research I read, or at least skimmed, the first four editions of the Tool Steels book. They are all relatively inexpensive to purchase on Amazon currently. You can read the 2nd and 3rd editions through open licenses online if you know where to look [14]. However, I cannot find a viewable version of the first edition online. Therefore, I scanned my copy of the 1st edition (don’t ever let it be said that I don’t put in work for this website!). I’m not 100% confident in the legality of posting it, but the author is long dead and the 2nd and 3rd editions can be read online legally so I’m not too worried. Also the 1st edition is useful primarily for historical reasons now and not really as a resource for tool steel knowledge. The link to the book, should you choose to read it, sends you to MediaFire as I don’t want to overload my hosting: https://www.mediafire.com/file/g403it9qmqd3gxu/Tool-Steels-1st-Ed.pdf

One more piece of fun history: my copy of the book is signed to A.G. McKenna in the front cover. The McKenna family was made up of metal workers, metallurgists, and business owners. The company Kennametal continues today. A.G. McKenna played a role in the early development of tungsten-alloyed high speed steel and T1, and this led to the founding of VASCO. In his thesis, James P. Gill claimed that A.G. McKenna was publishing articles about high temperature heat treatment even before Taylor and White, though he provides no citations for this. A.G. McKenna was never officially an officer of VASCO but his brother Roy McKenna became president of VASCO in 1915 [15], and the second edition of Tool Steels was dedicated to Roy. Whether A.G. McKenna asked for a copy of Tool Steels or if Gill signed it and gave it to him is probably impossible to determine now. I don’t know how it ended up on Amazon and sold to me. The inside cover lists the book as having been donated by David Earl McDaniel to the San Jose public library. I contacted the library and they told me that McDaniel was a poet and singer who collected thousands of books and donated them all to the library. Unfortunately he died in 1977, unmarried, and kept no records of where he got the books.

[1] Roberts, G A, and Robert A. Cary. Tool Steels. Beachwood, Ohio: American Society for Metals, 1980.
[2] Gill, James Presley. “High speed steel-its history, development, manfacture, metallography, and constitution, including an extended bibliography.” (1922).
[3] http://www.moxba.com/es/53/molybdenum-oxide/
[4] Uthoff, F. W., and S&T. Missouri. “Rollamo 1919.” (1919).
[5] https://www.summitdaily.com/news/climax-mine-a-brief-history/
[6] Kinzel, A. B., and C. O. Burgess. “Effect of Vanadium in High-speed Steel.” Trans. Amer. Institute of Mechanical Engineers100 (1932): 257-263.
[7] Gill, James P. “Alloy steel tool.” U.S. Patent No. 2,105,114. 11 Jan. 1938.
[8] Gill, James P. “Ferrous alloy.” U.S. Patent No. 2,174,281. 26 Sep. 1939.
[9] Theisen, W. “Hartphasen in Hartlegierungen und Hartverbundstoffe.” (1998).
[10] Hamaker, Jr John C., James R. Handyside, and Daniel H. Yates. “Ultra hard high speed steel.” U.S. Patent No. 3,259,489. 5 Jul. 1966.
[11] Gill, J. P., and Robert S. Rose. “Molybdenum High-Speed Steels.” Iron Age 148, no. 13 (1941): 33-35.
[12] “High Speed Cutting Materials.” Steel Processing and Conversion 32-33 (1946): 810.
[13] Automotive Industries 105 (1951): 158.
[13] https://www.hathitrust.org/
[14] http://www.referenceforbusiness.com/history2/92/Kennametal-Inc.html


Very cool article. I’m always so impressed by those that did the early research and doubly so when it stands the test of time.
I agree!
Well said Larrin. Netflicks documentary? A lot of Jame’s story could be depicted pictorially. Would make an awesome short bio story too.
I will get the petition started. It is pretty tough to put together much information on these old steel researchers. Metallurgists don’t care much about tracking their history, and developments of steel often stayed secret within various companies, and many of those companies are now gone.
Indeed, but its Hollywood, anything’s possible. Thought, we get your dad to narrate it and add some add hock info along the way.
Hi Larrin,
CPM 3V steel question.
Looking at the graph of the Effect of Vanadium in 0.64% – 0.8% Carbon with the hardness drop at about 3% vanadium, AND
at the CPM 3V composition of 2.75-3% Vanadium in 0.8% Carbon –
is it logical to expect CPM 3V to have worse edge retention compared to Elmax that has 3% Vanadium in 1.7% Carbon?
3V and Elmax both have appropriate levels of carbon to achieve 60+ Rc so it’s not really a question of sufficient carbon for hardness to compare their edge retention. 3V has a small volume (3-5%) of vanadium carbides whole Elmax has a much larger amount (15% or so) of somewhat softer vanadium-enriched chromium carbides. Fortunately we don’t have to guess which has better edge retention because Bohler Uddeholm did the CATRA testing already: http://www.bucorp.com/media/CATRA_Test2.pdf
Could you tell which line in the CATRA_Test2.pdf is for 3V steel?
Your saying that 3V has sufficient carbon (0.8%) to achieve 60+ hardness contradicts what you write in the High Vanadium Steels and the Gill’s Graph 12-97. “The issue with adding higher additions of vanadium was that beyond a certain point the hardness of the steel would drop significantly – 3% on the graph for 0.64%-0.8% carbon.”
Must be my lack of fundamentals that makes it illogical for me.
3V is PM 7.5-1.3-2.75 CrMoV
You’ll notice that in the article the reason for the drop in hardness was that ferrite was retained at high temperature so that the steel cannot be fully austenitized. The 17% tungsten has something to do with that because tungsten is a “ferrite stabilizer.” Also while the study cited is for 0.64-0.8%C, that means they were targeting approximately 0.7%C and carbon is a very strong “austenite stabilizer” so small differences in carbon matter. So 3V has slightly higher carbon, and a lower overall content of “ferrite stabilizing” elements with its 7.5%Cr and 1.3%Mo. So you get a plot at 1950°F for vanadium vs ferrite that looks like this:
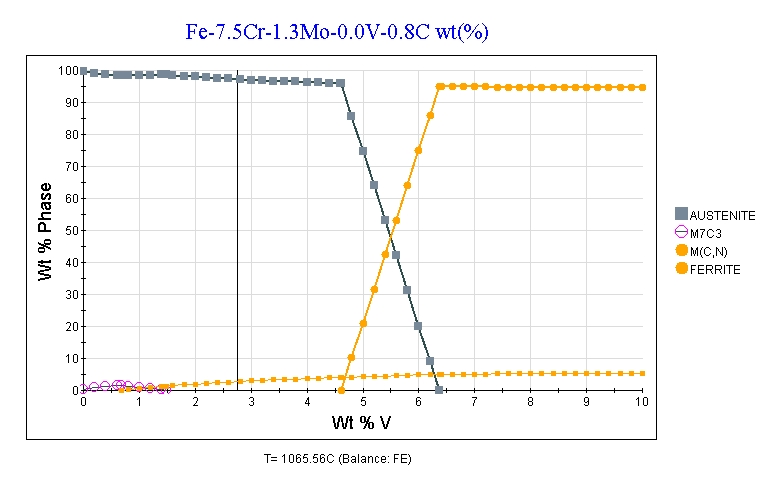
Now that’s clear even for an amateur like myself, thank you Larrin.
Appreciate your patience and talent to explain.
Thanks for the first edition scan! I notice that the subsequent editions were considerably expanded!
I have a copy of your book arriving tomorrow. Looking forward to studying it!