Thanks to Dinkma, Gavel John, and Roger Rozenberg for becoming Knife Steel Nerds Patreon supporters!
Low-Alloy Steel Testing
Over the past two years or so we have tested the toughness of many knife steels using a simple charpy impact test. You can read about the specifications of the test on this page. With the samples tested in this article I got a lot of help from Warren Krywko and Devin Thomas as well as donated steel from Alpha Knife Supply, Barmond Special Steels, and Achim Wirtz. This article focuses on the toughness testing performed on “low alloy” and carbon steels that are often used by forging bladesmiths. It is possible to forge high alloy steels, of course, though it does not appear to be very common even today.
We have previously published articles about the heat treatment of CruForgeV, 52100, 26C3, and 5160 which included a range of heat treatment variables to find the optimal set of properties. There is also an article on O1 though I am adding some information on O1 in this article as well. We have now tested enough of the available low alloy steels to talk about the group of steels more as a whole. Below is a summary of toughness data from the low alloy steels we have tested. All of the steels have a low Si content of 0.15-0.3% added for deoxidation so I removed that column from the composition table.


There are many things you may notice from the above data. For one, higher hardness generally means lower toughness. However, this is not the only factor that matters as can be learned if comparing steels at 60 Rc, for example, where the steels range all the way from ~8 ft-lbs all the way up to 50. If we compare the steels instead by carbon content of the bulk steel we get a somewhat more sensical trend:

The data has been simplified somewhat by only looking at steel that was tempered at 400°F but the trend is pretty consistent. Higher carbon leads to lower toughness. This is perhaps one of the most basic things known about steel. However, there are still some interesting things to analyze within this topic. For one thing, some steels fall above or below the trendline and exploring why may teach us something. For example, 52100 is significantly above the trendline. Why would it have comparatively high toughness? O1 and 1095 are a bit lower than the trendline. Why would those steels be lower in toughness?
Plate Martensite
One effect of carbon on toughness is the type of martensite that forms when quenching for high hardness. With low and medium carbon steels there is the formation of “lath” martensite which has relatively high toughness. When steels have about 1% carbon or more they form a different type of martensite called “plate” martensite which has lower toughness and sometimes there are “microcracks” that form during the transformation to plate martensite:

Image adapted from [1]

Image from [2]
However, in the region between 0.6 and 1.0% carbon there is an increasing amount of plate martensite leading to progressively reduced toughness. And hardness increases by only small amounts above about 0.6% carbon so in general we want to limit carbon “in solution” to be less than 0.6%:
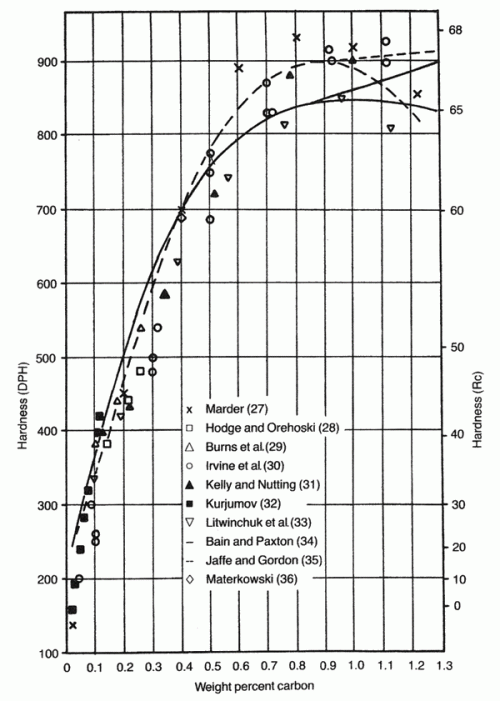
Image from [3]
When austenitizing steel, the process where the steel is heated to high temperature prior to quenching, there can be some carbide left undissolved so that not all of the carbon is put “in solution.” So a 1% carbon steel does not have to have 1% carbon “in solution” but could have less, with some carbide left in the steel for contributing to wear resistance. The amount of carbon in solution is controlled by the austenitizing temperature and the time at that temperature. Below I have shown the toughness of the low alloy steels vs a Thermo-Calc calculated carbon “in solution” at 1475°F. The real value would be somewhat lower as this calculation assumes an infinite hold time. 52100 has about 0.5% carbon in solution at 1500°F, for example. But the predicted value is likely to be strongly correlated with the “real” value despite some offset.

You can see that 52100, 1095, and O1 now appear more in line with expectations if we look at carbon “in solution” perhaps indicating that it is the amount of plate martensite which is partially controlling the toughness of the steels. Our two steels with 0.6% carbon in solution have by far the highest toughness. You may wonder what controls the amount of carbon in solution. After all, 52100 has slightly more carbon than 1095, but has much lower carbon “in solution.” Most alloying elements reduce carbon in solution for a given hardening temperature, though Mn is an important exception which can instead increase it. 52100 has 1.5% chromium which reduces the carbon in solution. You can read more about how this works in this article about 52100.
To test this plate martensite hypothesis further I performed another heat treatment on O1 using a lower than normal austenitizing temperature of 1425°F. This lower austenitizing temperature reduces the carbon in solution. I also included in the chart a heat treatment that was previously performed by Warren using 1550°F where carbon in solution is increased. Plotting those out show decent agreement with the earlier trend line. The 1425°F and 1475°F points are still a bit below the trendline but not incredibly so:

Carbides
Another factor that affects toughness is carbides within the steel. Higher carbon generally means greater amounts of carbide. Carbides are hard, brittle particles that contribute to wear resistance but are detrimental to toughness. Because of the complications with plate martensite the trends with carbide are not as “clean.” I drew in an approximate trendline, but the steels with lower carbon in solution (52100, 5160, 8670) have significantly higher toughness. And 1095 with its high carbon in solution has relatively poor toughness despite only a small amount of carbide.

If we generate a regression to predict toughness of low alloy steels, including the contribution of carbide does generate a better prediction, with an R2 value of 0.92 rather than 0.87 (1.0 is a perfect correlation).
Toughness (ft-lbs) = 107.5 – 105.9*C(in solution %) – 1.638*carbide(%)

The steels 1084 and 80CrV2 had very good toughness even relative to their carbon in solution, which is likely due to the fact that they have little or no carbide retained after heat treatment. I do not have micrographs for those two steels but I do for many of the others so that the carbide structure can be observed. The carbides are the white particles visible in the images. 5160, L6, 1095, and 8670 have very little carbide. The relatively high carbon in 1095 along with very little carbide after heat treatment is a good indication that most of the carbon is in solution which aligns well with our earlier predictions.

8670 Steel – 1525°F

5160 Steel – 1525°F

L6 Steel – 1550°F

1095 Steel – 1475°F
52100 steel does have some carbide which contributes to wear resistance, but O1 is the first steel in this series of micrographs which has a substantial amount of carbide and is also one of the steels that was below the trendline for carbon in solution vs toughness, perhaps indicating that its higher carbide content is contributing to reduced toughness:

52100 Steel – 1500°F

O1 Steel – 1475°F
With higher carbon steels we start to see more and more carbides and more interesting structures of them. CruForgeV is generally fine but has some occasional larger vanadium carbides. The larger carbides seem to be rare enough that they do not affect toughness too much, however.

CruForgeV – 1500°F – area 1

CruForgeV – area 2
The tungsten-alloyed steels V-Toku 2, 1.2442, and 1.2519 have 1-2% tungsten and 1-1.2% carbon which gives them a fine carbide structure but the higher carbide content still somewhat reduces toughness relative to the high toughness grades.

1.2442 – 1475°F

V-Toku 2 – 1475°F

1.2519 – 1525°F – area 1

1.2519 – area 2
The higher carbon and tungsten Blue Super and 1.2562 have coarser and somewhat odd carbide structures. The 1.2562 definitely has some larger carbides present, presumably either vanadium or tungsten-rich carbides which dissolve at higher temperatures. The 1.2562 also has a lot of carbide along grain boundaries which can be especially bad for toughness. The Blue Super is somewhat finer, presumably due to the reduced tungsten content relative to 1.2562, but still has an uneven microstructure. Perhaps with forging and re-annealing the microstructures could be improved somewhat vs heat treating from the “as-received” condition here.

1.2562 – 1475°F – area 1

1.2562 – area 2

Blue Super – 1475°F area 1

Blue Super – area 2
26C3 has relatively high carbon but is only alloyed with a small amount of chromium so it has a more even microstructure than the above high-tungsten steels. The carbides are still significantly larger than a steel like 52100, however. I would expect Hitachi White #1 to have a similar microstructure. 26C3 has relatively high carbon in solution and carbide volume but has decent toughness. I’m not sure what would make it have better toughness than a steel like O1 or 1095, for example. Maybe the superior cleanliness (low impurity elements) advertised by Uddeholm actually does lead to an improvement.

26C3 – 1475°F
Heat Treating to Maximize Toughness
Despite the reputation low alloy steels have for being “easy to heat treat” they can be very sensitive to temperature differences in terms of toughness. Heat treating steels to maximize toughness is a balance between the two factors described above: carbon in solution and carbide content. And grain size which has not been described because the steels were all austenitized in a controlled furnace at temperatures where grain growth is slow. In steels with lower carbon that tend not to have plate martensite, austenitizing at too high a temperature can lead to grain growth and a drop in toughness. With steels that have high carbon, plate martensite is likely to be an issue at a lower temperature than where grain growth is a problem.
Austenitizing Temperature
As one good example with balancing carbide, carbon in solution, and grain growth you could look at our study with 5160 toughness testing. Hardness increased by using 1500°F instead of 1475°F but was flat with higher temperature. Presumably more carbide was dissolved by increasing to 1500°F but because of the relatively low carbon content there isn’t a danger of plate martensite. Toughness was flat using 1475-1525°F but higher temperatures led to a significant reduction in toughness, presumably due to grain growth. Therefore the balance leads us to an optimum of 1500-1525°F.


Another steel that acts somewhat similarly is 52100 due to its relatively low carbon in solution. Higher austenitizing temperature led to increased hardness and toughness due to dissolving more carbide. At some temperature plate martensite starts to drop toughness. This led to an optimal austenitizing temperature of 1500-1525°F just like with 5160.


As described earlier in this article about O1, for steels with significant amounts of plate martensite it can often help to decrease the austenitizing temperature despite the higher carbide fraction. Reducing the austenitizing temperature means less carbon in solution, less plate martensite, and often an improvement in toughness. Below shows a summary of O1 heat treatments which indicates the austenitizing temperature (1425, 1475, or 1550°F) and tempering temperature (350, 400, or 450°F). The 1550°F austenitize included a cryo treatment but the others heat treatments did not. You can see that even when tempered to a similar hardness the lower austenitizing temperatures led to improved toughness:

With CruForgeV toughness had a consistent hardness-toughness balance when austenitizing between 1450 and 1500°F, but using 1550°F led to a dramatic reduction in toughness, probably due to plate martensite though maybe grain growth also contributed.

To provide a very loose recommendation, steels with higher than 0.85% carbon and less than 0.75% chromium benefit from 1475°F or below. This has to be balanced against ensuring the steel has been fully austenitized (no remaining ferrite) and that the steel has sufficient hardenability to fully harden during quenching. Perhaps 1400 or 1425°F would be a reasonable lower bound but that would require experimentation with the chosen steel.
Steels with less than 0.85% carbon or more than 0.75% chromium can often benefit from austenitizing a bit higher, such as 1500-1525°F.
Cold Treatments
Contrary to popular belief, low alloy steels can definitely have retained austenite present in them with normal heat treating, and cryo or dry ice treatments will affect the final properties. You can see this in the earlier 52100 data showing an increase in hardness but a decrease in toughness by using liquid nitrogen after the quench. The high carbon in solution with many of these steels means that retained austenite is likely, and only some of it is converted to martensite during tempering at 400°F. You can read more about cryogenic processing in these articles: Part 1, Part 2, and Part 3. Those articles describe how cryo works, whether it improves wear resistance and toughness, and what the pros/cons are for performing a cold treatment.
How Do You Know if There is Plate Martensite?
Plate martensite and excess retained austenite go hand in hand. Almost every published micrograph of plate martensite shows substantial amounts of retained austenite. Excess retained austenite can be seen by a drop in hardness with increasing austenitizing temperature. Therefore, I recommend performing a range of heat treatments with different austenitizing temperatures, quenching in a fast oil, and measuring the hardness. Don’t use cryo for this process as then the hardness drop will be pushed to higher retained austenite contents. Below shows an example with three different low alloy steels:

1.2519 is an example of a steel with a significant chromium content like 52100 where the hardness did not drop from excess retained austenite even at 1525°F. Similar behavior was seen previously in the hardness vs austenitizing chart for 52100. 1.2442 has only a small chromium addition so it reached its peak hardness at 1475°F but the hardness difference was small between 1450 and 1475; therefore the steel shouldn’t be austenitized higher than 1475°F and perhaps should be austenitized lower such as 1425 or 1450°F. 1.2562 with its very high carbon showed a drop in hardness even when increasing the austenitizing temperature from 1450 to 1475°F. So for this steel the max austenitizing temperature is instead 1450°F and maybe even lower. A lower carbon steel like 1084 or the previously shown 5160 instead reach a hardness plateau at some austenitizing temperature because the carbon in solution maxes out at a point where large amounts of austenite are not retained (though that does not mean zero retained austenite). With those steels it is probably best to austenitize at the temperature where the plateau begins and not higher where grain growth may occur.
The prior structure of the steel being heat treated, the time at austenitizing temperature, the speed of the quench, and the composition of steel (it varies even within a single grade) will all affect where the peak hardness is found. The prior structure of the steel matters, because when buying steel from different manufacturers their annealing process may be different. And if you forge some blades and use stock removal on others the heat treatment response is likely to be different as your annealing process is not going to be the same as the steel mill. And obviously if you change to a different annealing procedure the heat treatment response will be different. Keep austenitizing time consistent when developing hardness curves so that that factor does not change, though using different thicknesses of steel can confuse that a bit. Speed of the quench matters as slower oil quenches typically shift peak hardness to higher austenitizing temperatures. And different heats of steel will also show some variation as it may be on the high or low end for carbon content, chromium, manganese, etc.
I am not recommending against using cryo with low alloy steels, but only that it is easier to identify the point of excess retained austenite without cryo. So for this set of experiments when finding the upper bound of austenitizing temperature it is best to skip cryo, but if you want to minimize retained austenite and maximize hardness you can still use cryo for the final heat treating.
With high alloy and stainless steels using cryo with increased austenitizing temperatures doesn’t seem to be as big of a problem because those steels have lower carbon in solution. But the low alloy steels discussed in this article with such high carbon in solution are very sensitive to overaustenitizing and plate martensite.
Tempering Temperature
If the steel is not tempered at sufficiently high temperature the toughness is much lower. This seems to be more of a problem with high toughness steels like 5160 and 8670. For example, tempering 5160 at 350°F led to significantly reduced toughness relative to 375°F even though the hardness was only marginally higher.

A similar result was seen with 8670 with tempering at 175°F led to relatively high hardness but quite low toughness, lower than many of the high carbon steels when tempered to the same hardness. Some knifemakers make a mistake in assuming that a “tough” steel will do well at high hardness, when it is often better to switch to a higher carbon steel that is better designed for achieving that greater strength. We will look at more tempering temperatures with 8670 in the future to find the point at which the balance is off. So I typically recommend tempering steels no lower than 300°F though as can be seen with 5160 sometimes this minimum tempering temperature can be higher.

Tempering too high can also pose a danger, such as the 5160 tempering chart shown previously where a 450°F temper had somewhat less toughness than 400°F. While this drop in toughness may appear relatively small, there was also a drop in hardness, so the hardness-toughness balance has been reduced by using 450°F. A 400°F temper instead leads to both higher toughness and hardness for better properties overall. This effect is called “tempered martensite embrittlement” which you can read more about here. Larger cementite plates form when tempering at those higher temperatures which embrittle the steel. Here is another example, 52100:

Tempered martensite embrittlement (TME) starts around 450 or 500°F in low alloy steels (depending on the steel and austenitizing temperature) and you don’t know at which tempering temperature TME will start without quantitative toughness testing. So I typically recommend tempering no higher than 400-425°F without toughness data. 400°F tempering, in general, is a good starting point for most steels as it is unlikely to lead to either undertempering or tempered martensite embrittlement. 26C3 still showed an increase in toughness by using 450°F tempering. A 500°F temper with 26C3 did show a reduction in toughness, however:

L6 steel still showed good toughness with a 500°F temper. Perhaps its many alloy additions suppressed the formation of the cementite plates to even higher temperatures. Higher austenitizing temperatures can also shift TME to higher tempering temperatures so maybe the 1550°F austenitize used also contributed to the suppression of TME.

Other Heat Treatments
Here is a table summarizing the heat treatments used in toughness testing of the other low-alloy steels that have not previously been written about. All of these steels were heat treated from the condition supplied by the steel manufacturer. See the individual articles about other specific steels because some of those explored other prior conditions.

Did Toughness Match Expectations?
Many times when I show toughness charts there are some who are disappointed in the result for a favorite steel so they blame it on poor heat treating. It is impossible to rule out that a different heat treatment may lead to a better result, of course. But if I spent all my time trying different heat treatments every time this was brought up I wouldn’t be able to do anything else. But I have addressed a handful of specific cases below.
O1 had a bit worse toughness than I would have expected, but comparing with data from Crucible [4], Carpenter [5], Hitachi [6], and the old book Tool Steels [7], it appears that O1 toughness has never been rated particularly high. L6 with its high nickel content I also expected to have very impressive toughness though it didn’t reach all that high until it was tempered down to about 58 Rc. However, that behavior was also found in the Crucible datasheet [8] so that also fits with expectation. I expected the O7-like 1.2519 to perform more like 52100 with its high chromium content of 1.2% and therefore reduced carbon in solution. It didn’t do poorly but it was significantly worse than 52100. I used a 1525°F austenitizing temperature; maybe it needs a bit lower to boost toughness. The high carbon and tungsten 1.2562 was not expected to have stellar toughness based on the reported toughness of the similar F2 tool steel which I wrote about in this article about tungsten-alloyed steels. Most of these low-alloy steels have little or no reported toughness data, especially when it comes to comparing them all to each other. So this small database provides information that wasn’t available before. For knifemakers that would like to see their favorite steel do better I recommend heat treating the specimens and machining them to the simple rectangular dimensions listed here. Send them to me and I will test them.
Selecting a Steel
There is more to selecting a forging steel than just toughness, of course. Some steels are easier to forge than others; the steels with more alloying elements typically don’t move as well under the hammer. These steels have different levels of hardenability, where higher hardenability means that slower oils can be used (to reduce warping and cracking) and still achieve full hardness but also means that a “hamon” is difficult to achieve and also annealing is more difficult without a controlled furnace. Some steels require a longer time at the austenitizing temperature prior to quenching which makes heat treating by eye in a forge more difficult. However, I don’t recommend heat treating in a forge anyway due to how sensitive the steels can be to temperature as shown in the above discussion.
The other major decisions of steel selection are in terms of hardness and wear resistance. 8670 and 5160 offer decent hardness (58-60 Rc) in combination with very high toughness but they do not offer high wear resistance. Steels with higher vanadium or tungsten content such as CruForgeV, 1.2442, V-Toku 2, 1.2519, 1.2562, Blue Super, etc. all offer higher wear resistance because of the hard vanadium or tungsten carbides that are in those steels. In some cases knifemakers want to use higher hardness to avoid edge rolling with thin edges or to enhance edge retention. It would be better to use a higher carbon steel than to temper 5160 or 8670 at a lower temperature as discussed previously. In my steel recommendations article, I suggested using 8670 for “high toughness,” CruForgeV for “high wear resistance” and 52100 for balanced properties. Each of those can be substituted with other choices, such as using 5160 instead of 8670, or using one of the tungsten steels instead of CruForgeV. Some steels may be used for more specific applications like 26C3 for high hardness, thin cutting edges that are also easy to sharpen and offer some wear resistance. And for very high toughness applications like axe heads or throwing knives it may make sense to move to even higher toughness steel than 8670 such as 4340 or 4140.
Summary
The summary of this article could be given with the top two charts in the article which included the composition of the steels and then the measured toughness values for them. For some the toughness measurements are all they are interested in, and that’s fine. But there are other things to discuss as was written about in the rest of the article. In general, the higher the carbon in the steel the lower the toughness, with some exceptions like 52100. However, we then got into things on a deeper level including the effect of carbon in solution and carbide volume fraction (more is bad for both). And then some discussion of the effects of heat treatment on the steels where changing austenitizing or tempering temperatures can have strong effects on the toughness of the quenched and tempered steel. There are too many caveats to list here maybe. Austenitizing temperatures of 1500-1525°F are better for lower carbon or high chromium steels and 1475°F or somewhat below for steels with high carbon content. Don’t temper below 300°F or above 425°F. Cryo increases hardness but decreases toughness.
[1] Marder, A. R. “The morphology of martensite in iron-carbon alloys.” Trans. ASM 60 (1967): 651-660.
[2] Krauss, G., and A. R. Marder. “The morphology of martensite in iron alloys.” Metallurgical Transactions 2.9 (1971): 2343.
[3] Krauss, George. “Martensitic transformation, structure and properties in hardenable steels.” Metallurgical Society AIME,(1978): 229-248.
[4] https://www.crucible.com/eselector/prodbyapp/tooldie/ketos.html
[5] https://cartech.ides.com/datasheet.aspx?i=102&E=113&FMT=PRINT
[6] https://www.hitachi-metals.co.jp/e/products/auto/ml/pdf/cwts_b.pdf
[7] Roberts, G A, and Robert A. Cary. Tool Steels. Beachwood, Ohio: American Society for Metals, 1980.
[8] https://www.crucible.com/eselector/prodbyapp/tooldie/champloy.html


Great article. I really enjoyed this as I typically forge simple steels like these and this helps me a lot! Keep up the great work! I’d be happy to send you some steel to test!
Thanks Scott!
Fantastic, informative article — Thank You!
Very informative article. Wow! The 8670 steel is tougher than L-6, 5160 and Z-Tuff. So, it is comparable to the S7 steel toughness?
Before this, i thought 8670 steel is quite similar to 80CRV2 with a little higher in toughness. But from this chart, I noticed that 8670 is a different animal.
In Malaysia, most of the bladesmith use 5160 for long chopper knife or short sword. But 8670 steel looked like a better option now.
Also interested in this response!!!
Interesting comparison, didn’t expect 52100 to score that good. Apart from that it seems nickel steels are the most promising ones – although some alloys, not included here, have way higher nickel content like german 50NiCr13 (3.5%!). Would have liked to see data for 3V and AEB-L too. Not including them in an article about though steels is like writing an article about typical cutlery steels and leaving out VG10.
AEB-L and 3V are not low alloy steels so they were not discussed.
Hello Larrin.
I always enjoy reading your treatise.
One question is about O1 and CruForgeV.
I think these steels have little difference other than tungsten and vanadium, but their toughness seems to be very different.
It can be expected that 1095 of high carbon and low manganese has low toughness, but why is there such a difference between O1 and CruForgeV?
Does tungsten have any effect on toughness?
I’m sorry if Google Translate doesn’t make sense.
I’m not good at English.
The Mn and V contents are quite different between O1 and CruForgeV. Maybe it’s enough to affect carbon in solution. Or maybe the forging/normalizing/annealing process we used with CruForgeV led to good results. O1 was tested as-is from the steel supplier.
Thanks for all the great info and data!
Question:
Did you test 9260 steel?
And if so how did it do in the testing?
Thanks again.
I have not tested 9260
Okay.
Would like to see how it compares to L6, 5160, and S5.
Thanks
Hello Larrin,
I want to comment your sentence : “Another steel that acts somewhat similarly is 52100 due to its relatively low carbon in solution. Higher austenitizing temperature led to increased hardness and toughness due to dissolving more carbide”
You posted, on other topic, the hardness of each phase (martensite, Fe3C, vanadium carbides, Nb carbide, etc) and martensite has lower hardness than any other carbide. How can low C steel have higher hardness with high austenizing temperature (=less carbide)?
Thank you for your help
The hardness of the steel is controlled primarily by the hardness of the martensite matrix not the hardness of the carbides or the volume of the carbides.
Great article, but i dont understand one thing, the time of temper? for example in the last chart in the 1095 temper at 400F the hardness is 60HRC what time is nedeed at that temperatura?
Performing a 2 hour temper twice is typical.
Its really interesting to see 1095 used so often when it seems like it is a bad mix of elements in general. I am curious about a steel being sold pretty cheap online, 50100. It is basically 1095 (with tighter Sulphur restrictions) and .5% chromium. Based on my reading of the above article i would expect the Chr to pull C out of solution and reduce the formation of plate martensite and increase the toughness of the steel. Does that sound accurate?
The 0.5% chromium definitely changes the behavior. Toughness could be improved.