Thanks to anteck7 and Aaron Lee for becoming Knife Steel Nerds Patreon supporters!
History of AEB-L and 13C26
Tracking down the history of AEB-L was surprisingly difficult. The Uddeholm website claims that AEB-L was patented in 1928 [1]; however, that is not entirely truthful. Uddeholm did patent a stainless steel in 1928 [2], which was named AEB, and later AEB-H to differentiate it from AEB-L. This was a very early stainless steel, so its development and patent needs to be viewed in that historical context. You can read about the development of stainless steels in this article. The AEB patent was for 0.7-1.1% carbon, 10-16% chromium, and 0.75-2.0% manganese. The original Brearley and Haynes stainless steel patents were still in effect; they got around them by using a higher carbon content than the Brearley patent (had a 0.7% max), and by claiming that high Mn led to improved corrosion resistance (it actually doesn’t). The nominal composition of AEB became 1% carbon and 13.5% chromium, which gave it a relatively large carbide structure compared to AEB-L, but it did see some use as a razor blade steel.

AEB-H/19C27 microstructure with some large carbides

13C26 (same as AEB-L) with very small carbides
Wilkinson first released stainless razors in Britain in 1956. In the early 1960’s Wilkinson sword introduced stainless razors on a broad scale, which quickly gave them a competitive advantage [3]. Gillette patented the polymer coating on razor blades which made the shave smoother and protected the blade from corrosion [4], which mentioned stainless steel as an option in 1959, but had not released any stainless razors. Schick, American Safety Razor, and Gillette all released their own stainless steel razors in the years shortly after Wilkinson [3]. Stainless razors were so disruptive that Gillette, despite being the largest razor manufacturer in the world, had dropped 8% in 1963 and a further 11.5% in 1964. AEB-L was not used in these early razors. Wilkinson’s patent on stainless razors listed Uddeholm SS716 (0.35C-13.5Cr-1.0Mo) and the previously mentioned AEB, but AEB-L was not mentioned [5]. Uddeholm did, however, produce the majority of the stainless steel for razors, with 85% made by Uddeholm in 1961 [6].

The development and transition to AEB-L from AEB is hard to track. AEB-L has reduced carbon relative to AEB (0.65-0.7% C) which gives it a much finer carbide structure, while being capable of much higher hardness of lower carbon steels like Uddeholom 716. The finer carbide structure makes it capable of better sharpness than AEB, though superior fine blanking (stamping without cracking), and improved cold rolling performance were likely significant factors. I found a reference to an Uddeholm steel with 0.5%C and 13%Cr used in microtome knives (require very high sharpness) in 1955 [7], but I can’t find a name for this steel and it doesn’t seem to have been used in razors. A Gillette patent filed in 1962 references only AEB as an example of a stainless razor steel [8], but in a patent filed in 1964 it references both Uddeholm AEB and Sandvik 12C27 [9]. 12C27 still exists today but may have served as a precursor to Uddeholm AEB-L and Sandvik 13C26. 12C27 has somewhat less carbon (0.6%C) and somewhat higher chromium (14%), and its properties are close to AEB-L and 13C26. I believe that the good performance of 12C27 led to AEB-L and 13C26 which were adjusted to have higher hardness. The German 1.4034 steel (0.45%C, 13%, Cr) existed by at least 1957 [10], which may have also served as a stepping stone to the higher carbon 12C27. Uddeholm filed for patents of processing methods for decreasing the final carbide size of steel in the late 1960’s [11][12], both for improving cold rolling performance (avoid cracking) and toughness. Those patents included a composition for AEB-L. So the timeline as I see it is that Wilkinson started producing stainless razors in Uddeholm AEB in 1955-1956, Sandvik 12C27 saw some use by 1964, followed by Uddeholm AEB-L in the mid or late 1960’s, and Sandvik 13C26 by 1975 [13].
Use of AEB-L and 13C26 in Custom Knives
12C27 and AEB-L have been used in production knives for many years, particularly in Europe, due to their very good blanking properties, which is significantly cheaper than saw cutting or waterjet cutting. However, the steel used in those knives was not frequently advertised so finding an early date for them is not easy. Sandvik and Uddeholm sold some steel to custom knifemakers but had minimum order sizes which were prohibitive to most. My father, Devin Thomas, began using AEB-L in the early 1990’s in his stainless damascus. He had been looking for a steel available in thin strip to minimize the number of forging and re-welding operations. Wayne Goddard recommended that he look into AEB-L. Advertising the damascus as being produced in AEB-L was a roadblock as the steel was not widely known, and the composition was not exciting with only 0.68% carbon. Up until 2005 popular articles summarizing types of blade steel stated that AEB-L is similar to 440B stainless, which has a poor reputation [14]. In March 2005, John Verhoeven released “Metallurgy for Bladesmiths” where he wrote about AEB-L as being an ideal blade steel due to its very fine carbide size and high potential hardness [15]. Also in 2005, I wrote a short summary about AEB-L for DevinThomas.com to promote the virtues of AEB-L where I attempted to describe why it is different than 440A and 440B [16]. In 2006 Roman Landes published Messerklingen und Stahl where he gave AEB-L very high scores for sharpness, ease in sharpening, toughness and edge stability. The steel continued to see little use in custom knives, in part because it was not regularly available at knife supply companies.
By 2004, Kershaw began to use Sandvik 13C26 [18], and more broadly in 2007 [19] due to its high potential hardness and very good blanking performance. Sandvik began attempting to get into the knife market to a greater extent at this point, and began selling their 13C26 through Admiral Steel in the US in 2007 [20]. Sandvik developed 14C28N for Kershaw to improve the corrosion resistance of 13C26, and those knives were being sold by 2009 [21]. You can read about what the nitrogen addition to 14C28N does in this article. The 13C26 steel was used in relatively inexpensive consumer knives where returns are likely if any rust is observed, leading to the development of 14C28N. By 2010 AEB-L was also offered in good sizes for knives through supply companies [22] and its popularity grew to today where it is relatively common in custom knives.
Design of AEB-L
To talk about why the development of AEB-L was significant we have to talk about what steels were available at the time. Here is a partial list of pre-existing stainless steels:

The requirements for achieving high performance in razors or custom knives means that we need relatively high hardness, preferably at least 60 Rc. 420, 440A, and 1.4034 fail that test, with 1.4034 coming the closest to achieving that goal. 440C and particularly AEB can reach high hardness due to the significantly higher carbon content (~1%). We would also like to have good wear resistance for slicing edge retention. 420, 440A, and 1.4034 again fail this test due to low hardness. However, 440C and AEB with their high hardness and large volume of chromium carbides means that they have high wear resistance. We would also like a steel with high toughness. 420 and 1.4034 with their fine microstructure do have high toughness, while 440C and AEB have relatively low toughness due to large carbides. So on one end we have steels that have a fine microstructure and high toughness but low hardness, and on the other end we have steels with high hardness and wear resistance but low toughness. For razors, 440C and AEB are not desirable because they are difficult to fine blank without cracking and to sharpen to the extreme sharpness that razors require. And the other three steels do not have sufficient hardness for good performance in a razor.
There seem to have been two directions that metallurgists had gone with previous stainless steels, either the high chromium 17% approach or the low chromium approach with 13%. 420 with 0.3% carbon and 13% chromium was the first commercial stainless, and when carbon was raised to increase hardness they thought that chromium also needed to be increased to maintain corrosion resistance, which led to 440A and 440C. AEB had significantly less corrosion resistance than those other steels (around 10.5% Cr in the chart below), despite the claim that Mn would improve corrosion resistance. As expected, with higher chromium, the “chromium in solution” that contributes to corrosion resistance is higher. If the chromium is not “in solution” it does not contribute to corrosion resistance. You can read more about chromium in solution and corrosion resistance in this article.

The reason that the increase in carbon decreases the amount of chromium in solution is because more chromium carbides are formed. More carbon means the steel wants to form more carbide. So if you want to add more carbon to the steel, corrosion resistance can be maintained if the chromium content is also increased. However, more chromium also reduces the amount of carbon that goes in solution to contribute to hardness. If the carbon is tied up in carbides then it is not contributing to hardness. You can read about how carbon increases hardness in this article. To regularly reach 60+ Rc we probably want about 0.5% carbon in solution, which for a steel with 13% bulk chromium requires about 0.7% carbon on the chart below. For a steel with 17% bulk chromium, more than 1.1% carbon is required to reach the same carbon in solution:

So because of the effect of higher chromium, carbon in solution only increases from 0.3% to 0.37% by moving from 420 to 440A, despite 440A having 0.4% higher carbon. Where does the extra carbon go? To carbides. More chromium means the steel wants to form more chromium carbide. So more carbon and more chromium both lead to more carbides. That can be seen in the following chart:

More chromium carbide means better wear resistance, but at some point the greater amount of carbide and larger carbide size will mean that toughness and blanking performance is poor. During casting of the liquid steel, different phases can form at different temperatures. The typical high temperature steel phase “austenite” can form as well as chromium carbides. The higher the temperature is that the carbides form the larger they will be, and that temperature is also controlled by the carbon and chromium content. I have plotted the temperature at which the steel solidifies to austenite (“solidification”) and also the temperature at which the chromium carbide begins to form for both 17% and 13% chromium:


The higher the temperature of carbide formation, the larger the carbides are, particularly when carbide forms at the same temperature that the steel completes solidification. When the carbide formation and solidification temperature are the same that leads to the formation of “ledeburite,” a combination of carbide and austenite, which is difficult to break up during forging leading to large chromium carbides. You can learn about ledeburite and how powder metallurgy helps in this article. If we subtract the carbide formation temperature from the solidification temperature, the difference correlates very well with carbide size:

Though the thermodynamic software predicts that the carbide formation occurs at a lower temperature than solidification for all but the “0” point on the chart above, segregation of elements like carbon and chromium means that it is more and more likely that ledeburite is formed the smaller the predicted difference between solidification and carbide formation. At the top end is 440C with carbides over 10 microns, and AEB-L on the low end with carbides of one micron or less:

440C has large carbides

13C26 (same as AEB-L) has tiny carbides
So the design of AEB-L is essentially the following: maximize hardness and wear resistance while still maintaining very tiny carbides and good corrosion resistance. If we take the same plots of carbon and chromium in solution, we can make another view that tells us the maximum combinations we can achieve with different carbon and chromium contents:

Any place in orange on the chart means that all of the carbon and chromium is in solution at 2000°F. So a steel with 0.4% carbon and 11% chromium at 2000°F would have no carbide and would have 0.4% and 11% chromium in solution. The line marked “max carbon-chromium” is the point above which some carbide will form (the blue area). An infinite amount of carbon and chromium cannot be in solution because carbides will form. So with 0.4% carbon in solution the maximum amount of chromium in solution is about 15.5%, and with 1% carbon it is 6.5%. The farther away the steel composition is from the max line the more carbide that has formed. So first we need to pick a place along the line that gives the balance of hardness and corrosion resistance we want. To achieve high hardness, we should have around 0.5% carbon in solution, as that will give us about 62 Rc as-quenched so that we can temper down to 60 Rc:
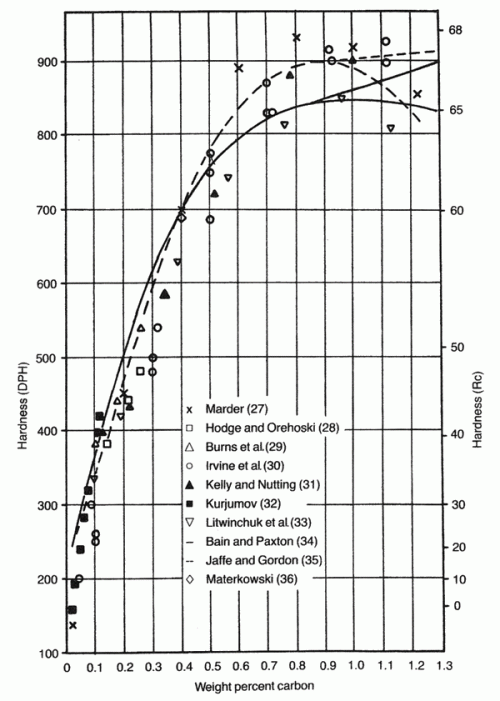
The 0.5% carbon point on the max carbon-chromium line also gives us around 12-12.5% chromium in solution which is sufficiently high for good corrosion resistance, as shown in this chart showing the amount of weight loss (corrosion) for different levels of chromium:

Then we can set how much carbide we want by the distance from the “max carbon-chromium” line. We want a relatively small fraction of chromium carbide to provide wear resistance, while not having so much that we have large carbides like 440A, 440C, and AEB. You can see the position of the previous stainless steels relative to the diagram, along with the “tie lines” for AEB-L, 440A, 440C, and AEB showing the carbon and chromium in solution. The length of the tie line is an indication of how much carbide is present.

So AEB-L is an expertly designed steel, balancing high hardness with good corrosion resistance, and maxing out the amount of carbide that still maintains high toughness, sharpness, and blanking performance for razors. Those qualities make it a good knife steel in many ways as well.
Heat Treatment
I did some simple heat treatment experiments with AEB-L recently, measuring hardness and retained austenite with different austenitizing/hardening temperatures. For each temperature I held for 15 minutes prior to a plate quench, followed by either liquid nitrogen for 30-60 minutes, my household freezer for 30-60 minutes, or no cold treatment. As expected, the liquid nitrogen treatment cryogenic treatment led to the highest hardness, followed by the freezer, then room temperature. Some have claimed that a freezer isn’t cold enough to do anything. You can read about cryo, freezer, and other cold treatments in this article.

Using the freezer or liquid nitrogen also raises the austenitizing temperature that leads to maximum hardness: 1925°F with room temperature, 1950°F with the freezer, and 1975°F with liquid nitrogen. The peak hardness was increased from 62.2 Rc with room temperature to 62.8 Rc with the freezer, to 64 Rc with liquid nitrogen. Higher austenitizing temperature means more carbon in solution for higher hardness, but more carbon and chromium in solution also increases the amount of retained austenite. At some amount of retained austenite, usually in the range of 15-20%, the soft austenite begins to affect the bulk hardness of the steel and the hardness drops. This was also seen in retained austenite measurements of AEB-L:

Interestingly, the peak hardness is obtained with less retained austenite when using liquid nitrogen than when cooling only to room temperature, about 12% for liquid nitrogen (1975°F) and about 22% for room temperature (1925°F). Perhaps that means that strength is higher when using cryo even when at the same hardness. Learn why in this article about why rockwell hardness can be misleading. I also generated a tempering curve for 1925°F with room temperature and 2000°F with cryo:

The hardness is increased by using low tempering temperatures, you can learn why in this article on tempering. Some of that hardness is maintained even with 300°F tempering, where nearly 65 Rc was reached with cryo. 300°F tempering temperature is the minimum that I typically recommend for heat treating with good toughness.
Some low alloy steels can lose some retained austenite during tempering at temperatures of 400 or 450°F, but the high chromium content of AEB-L means that retained austenite was flat because the loss of austenite was suppressed:

My values for hardness are similar to, though somewhat higher than, those reported by Uddeholm [23] and Sandvik [24][25]:


Toughness
Along with Michael Drinkwine we recently did a study on AEB-L toughness, looking at a range of heat treatment parameters. You can read about our toughness testing procedure here. One thing we wanted to look at is the effect of a prequench. A prequench is similar to the “multiple quench” or “triple quench” used in low alloy steels for grain refinement. We tested a triple quench with CruForgeV and found no benefit to toughness in this article. And I have previously written about prequenches in this article. Multiple quenches with high alloy steels have been found to lead to “discontinuous grain growth” leading to larger grains and poorer toughness. However, if the first quench is performed from a lower austenitizing temperature, grain refinement has been observed with D2 and M2 tool steels:

In the chart above you can see that a double austenitize and quench from 1010 °C (1850°F), the common temperature for D2, there was no grain refinement. However, if the first austenitze and quench was performed from 870, 925, or 980°C (1600-1800°F) then the grain size was smaller. No toughness numbers were included, however.
So for AEB-L we tried prequenches of 1650, 1750, and 1850°F, all for 10 minutes and plate quenched, followed by an austenitizing at 1925, 1975, or 2025°F, plate quenched, and given a 30 minute cryo treatment. They were finally tempered twice for an hour each time at 350°F unless otherwise indicated.
Unfortunately, prequenching does not appear to have improved toughness. Toughness was flat with the exception of the 1750°F prequench which had lower toughness because the hardness was increased for reasons unknown. There may have been a small improvement using 1850°F but that could very well be experimental noise.

Comparing the 1750°F prequench across different austenitizing temperatures, it is seen that the increase in hardness was consistent. The increase was smallest at 2025°F, likely because retained austenite lowered hardness at that temperature:

There was a corresponding decrease in toughness because of the higher hardness from the 1750°F prequench. However, the greatest decrease in toughness was with 2025°F austenitize despite having very similar hardness. That may indicate that the prequench led to either grain growth or excessive carbon in solution for a decrease in toughness when over-austenitized:

When plotted vs hardness this same behavior is seen, where toughness is primarily controlled by hardness whether prequenched or not, expect for the case of 2025°F austenitizing which I will discuss later. However, in the cast of the 1750°F prequench the hardness-toughness balance is a little bit better than the non-prequench, and 1650 and 1850°F prequench heat treatments. There is enough scatter in toughness testing that this may have not been a real improvement. On the other hand, a small decrease in grain size fits this degree of toughness improvement.

When looking at tempering, increase from 300 to 350°F leads to a decrease in hardness and an increase in toughness, as expected. There is no big drop off in toughness by using 300°F, so it is probably safe to got that low for tempering temperature with this steel, if the extra hardness is desired:

Decent toughness (11.8 ft-lbs) was obtained even with 1975°F-300°F giving 64 Rc. Plotting hardness vs toughness for all heat treatments shows that hardness was the primary controlling factor for toughness as long as the steel was not over-austenitized:


Heat Treatment Recommendations
Therefore a range of hardness-toughness combinations can be achieved by adjusting heat treatment parameters. To maximize hardness, use 1975°F for 15 minutes along with cryo and a 300-350°F temper. To maximize corrosion resistance, use the same 1975°F and temper down to the desired hardness, up to 600°F or so. For high toughness use 1925°F for 15 minutes along with a 350°F temper or higher. Do not austenitize above 2000°F or toughness will drop somewhat. A prequench from 1750°F may increase hardness slightly, and also a small increase in the toughness-hardness balance. Dry ice likely gives similar results to liquid nitrogen, though I have not tested it. If dry ice or liquid nitrogen are not available, then use a freezer along with a 1925-1950°F austenitize.
Toughness Compared
These toughness values are slightly lower than a previous test we did with AEB-L using a 1950°-350°F heat treatment using cryo but no prequench. However, that difference can probably be explained by a different batch of steel or perhaps just experimental variability. That higher toughness datapoint is the separate floating green triangle along with the simplified dataset from the toughness testing with Michael Drinkwine. Regardless, AEB-L still scores very well for toughness relative to other steels, particularly stainless steels, due to its small volume of very tiny carbides. You can read how carbides affect toughness in this article.


In terms of stainless steels AEB-L beats everything except for possibly “new steel” which was discussed briefly in this article on niobium steels. AEB-L at 62.2 Rc matches the toughness of 59.8 Rc CPM-154. And at 64 Rc AEB-L is still tougher than a range of steels including D2, M390, and Elmax at lower hardness. In terms of non-stainless steels AEB-L is slightly better than 52100 and Z-Wear, an impressive showing, though it does not quite match the very tough 8670. At 64 Rc AEB-L is approximately equal to CruForgeV at the same hardness. These excellent toughness values means that AEB-L can be heat treated to high hardness for high “edge stability” for thin edges that need high strength. Maximizing hardness also means that wear resistance is maximized, of course. Lower hardness can be used for applications that require very high toughness.
Slicing Edge Retention
The factors that control slicing edge retention are described in this article on CATRA testing. In terms of knives, edge geometry is the most important factor for edge retention. In terms of steel, the hardness of the steel, the hardness of the carbides, and the volume fraction of the carbides control edge retention. High hardness steel, and a large volume fraction of very hard carbides is best for high edge retention.
After heat treatment AEB-L has around 4-6% chromium carbide. That is significantly less than lower toughness stainless steels like 440C, CPM-154, Elmax, M390, etc. which all have 16%+. That is why AEB-L has very high toughness, but it also means that the wear resistance and slicing edge retention is not going to match those high carbide stainless steels. However, because chromium carbides are harder than the iron carbide cementite in low alloy steels like 1095, O1, and 52100, AEB-L does slightly exceed the edge retention of those steels. That was confirmed in CATRA testing by Verhoeven where he compared AEB-L with 52100 and 1086 [26]. We previously compared the CATRA edge retention of AEB-L with 154CM and AEB-L/154CM damascus, which I first wrote about in this article. This relative value allows us to compare the edge retention of AEB-L with other steels using results reported by Crucible [27] and Bohler-Uddeholm [28]:

The values above are the edge retention numbers relative to 440C, hence why it is 100, meaning 60 Rc AEB-L has approximately 55% of the slicing edge retention of 440C at 59 Rc. Heat treating AEB-L to higher hardness would increase the slicing edge retention value but to what extent is not known. Using the previously calculated effect of hardness on CATRA edge retention, I would expect AEB-L to reach as high as 75 on the chart above when at maximum hardness.
Balance Between Toughness and Edge Retention
Plotting toughness vs edge retention shows that AEB-L is by itself compared to many of the commonly available stainless steels:

AEB-L has much higher toughness and correspondingly lower edge retention than many other common stainless steel options. Other stainless steels not on the chart (S35VN, Vanax), are unlikely to come close to the toughness of AEB-L because they also have very high carbide/nitride volume (13%+). AEB-L is really a steel that is more similar to low alloy steels like 52100 and O1, where they have very good toughness and average wear resistance. This sets apart AEB-L from many other stainless steel options that are all kind of in the same ballpark for edge retention (high) and toughness (low). It is true that the powder metallurgy process in steels like CPM-154 does improve toughness but the high carbide volume fraction limits toughness. The very fine carbides and low volume fraction of them means that the toughness of AEB-L is very high.


The carbide size of AEB-L (top) is much finer than even the powder metallurgy CPM-154 (bottom)
Edge Stability, Cutting Ability, and Edge Retention
Thin edges sharpened to acute angles cut much better than thick edges. This is called “cutting ability” which is the energy required to cut through something. This is different than sharpness. An axe can be sharpened to very high sharpness but the energy required for cutting remains relatively high. A knife with high cutting ability, even while dull, will keep on cutting depending on the material being cut. You can read about the differences between sharpness and cutting ability in this article. Thin edges with acute angles need very high strength (hardness) to avoid rolling and deformation, and high toughness to avoid chipping. This combination of strength and toughness is called “edge stability,” which you can read about here. AEB-L with its high toughness has very good “edge stability” meaning it is very well suited for high cutting ability edges. Thin edges do not just cut better, however, they also have much better edge retention. That means that when AEB-L is used in knives with thin, acute edges where it excels relative to the more wear resistant steels that have poor toughness, the edge retention will match or exceed those more wear resistant steels. So AEB-L knives can have both better cutting ability and edge retention than more wear resistant steels when used with an edge geometry where it excels.
Grinding, Polishing, and Sharpening
Another area where AEB-L is much closer to low alloy steels than to other stainless steels is in grinding, polishing, and sharpening. The relatively low wear resistance means that AEB-L excels in this area. It is very easy to grind and finish for the knifemaker, and very easy to sharpen for the end user. For those users that would like to trade some edge retention for ease in sharpening, AEB-L is a very good choice. For knifemakers that need to put a high finish on a knife or to minimize hand sanding time, AEB-L is better than even 440C or CPM-154. The cleanliness of AEB-L is also very good which means that impurities are unlikely to affect the finish.
Cost
The cost of AEB-L is usually quite low compared to many other stainless steel options. This is in part because it is produced through conventional metallurgy techniques rather than expensive powder metallurgy. Low requirements for time in finishing/grinding, belts, sandpaper, etc. also keeps costs low when using AEB-L in knives.
Recommended Applications
With its high toughness and good potential hardness, AEB-L excels in applications requiring thin edges like razors, fine slicing fixed blades and folders, and kitchen knives. Especially in knives where corrosion resistance is desired. It also does well in knives that require high toughness that will be subjected to hard use. It is not a good choice for applications that require very high slicing edge retention or in very corrosive environments like salt water. It is an especially good choice for those that prefer simple carbon steels and low alloy steels but would like to try a stainless steel. AEB-L will behave more similarly in grinding, polishing, and end user properties than many other high wear resistance stainless steels.
Legacy of AEB-L and 13C26
I think the main value of AEB-L was that it was another step in the understanding how to balance the properties of stainless steels. Metallurgists learned more about how to balance composition and processing to control the carbide size of stainless steels to keep it fine and to maximize properties like toughness and sharpness. Sandvik used 13C26 as its base product when developing the improved corrosion resistance version of 14C28N. That allowed them to use the same principles to achieve high toughness with a small amount of chromium carbides and nitrides for wear resistance while maintaining good blanking performance for inexpensive consumer knives. The newer Nitro-V also is very similar to the original AEB-L showing that as a starting composition it is still a useful baseline. While it took quite some time for AEB-L to become a common knife steel among custom knifemakers, its use is now ubiquitous.

Summary and Conclusions
Uddeholm AEB-L was developed in the 1960’s, with the identical Sandvik 13C26 coming shortly after. Both were developed as stainless razor blade steels which required a combination of high hardness and sharpness, along with good wear resistance, blanking, cold rolling, and corrosion resistance. Those properties also make a good knife steel for many applications. The good properties were obtained by carefully balancing the carbon and chromium content to achieve high hardness and corrosion resistance without forming too many carbides. Heat treatment tests show that AEB-L can achieve 63+ Rc for high strength and edge stability. Toughness testing shows that AEB-L matches or exceeds other common steels, even non-stainless steels. Prequenching from 1750°F may have slightly improved the hardness-toughness balance. Edge retention of AEB-L is average; better than low alloy steels like 52100, but not as good as high wear resistance steels like CPM-154 and S30V. However, the high edge stability of AEB-L means it can be used in knives with thinner edges where it has both superior cutting ability and edge retention to steels with more wear resistance but poorer toughness. AEB-L works well in knives with thin edges for fine slicing, as well as in larger knives requiring high toughness. AEB-L excels when it comes to low cost and ease in grinding, finishing, and sharpening. AEB-L works very well as a stainless alternative to low alloy steels. The steel has had a strong influence on other stainless steels serving as the baseline for products like 14C28N and Nitro-V. The use of AEB-L in custom knives is now quite common as its popularity has grown significantly in recent years.
[1] https://www.uddeholmstrip.com/files/va_folder_6sid_razorscalpel_high.pdf
[2] Veges, Arved Eduard Gaston Theo. “Manufacturing edge tools and special composition of steel for same.” U.S. Patent 1,644,097, issued October 4, 1927.
[3] “The Blade Battle,” Time Magazine, January 1965.
[4] Fischbein, Irwin W. “Razor blade and method of making same.” U.S. Patent 3,071,856, issued January 8, 1963.
[5] Deacon, Roger Frederick. “Safety razor blades.” U.S. Patent 3,425,877, issued February 4, 1969.
[6] Western Machinery and the Western World, vol. 52, 1961.
[7] Ekholm, R., O. Hallén, and T. Zelander. “Sharpening of knives for ultramicrotomy.” Experientia 11, no. 9 (1955): 361-362.
[8] Harvey, Alter. “Razor blade having a coating of a cured solid hydrocarbon polymer on its cutting edge.” U.S. Patent 3,071,858, issued January 8, 1963.
[9] Fischbein, Irwin W. “Razor blade and method of making same.” U.S. Patent 3,518,110, issued June 30, 1970.
[10] Hans, Malzacher. “Method of producing articles having a cutting edge portion and consisting of stainless chromium steel.” U.S. Patent 3,116,180, issued December 31, 1963.
[11] Jakenberg, Klas-Erik. “Method in the manufacture of stainless, hardenable chromium-steel strip and sheet.” U.S. Patent 3,660,174, issued May 2, 1972.
[12] Omsen, Arne Haraldsson, and Bertil Ring. “Method of treating steel.” U.S. Patent 3,615,905, issued October 26, 1971.
[13] Bergquist, B. et al., SANDVIK 13C26 Stainless Razor Blade Steel, Bulletin 66-4E, Steel Research Centre, Sandvik, Sandviken, Sweden, Oct. 1975, p. 1.
[14] https://web.archive.org/web/20040208155352/http://ajh-knives.com/metals.html
[15] Verhoeven, John D. “Metallurgy of steel for bladesmiths & others who heat treat and forge steel.” Iowa State University (2005).
[16] https://web.archive.org/web/20060213062223/http://www.devinthomas.com/pages/faq.html
[17] Landes, R. “Messerklingen und Stahl.” Aufl. Bad Aibling: Wieland Verlag (2006).
[18] https://www.bladeforums.com/threads/kershaw-onion-question.299530/#post-2553240
[19] https://www.bladeforums.com/threads/420hc-stainless-steel-or-sandvik-13c26-cpm-d2.606650/#post-6300374
[20] https://www.bladeforums.com/threads/sandvik-steels-and-reps-at-the-blade-show.478482/
[21] https://www.bladeforums.com/threads/420hc-stainless-steel-or-sandvik-13c26-cpm-d2.606650/#post-6301962
[22] https://web.archive.org/web/20100416090322/http://www.alphaknifesupply.com/bladesteel.htm
[23] Out of print AEB-L datasheet, I’ve hosted it here: https://knifesteelnerds.com/wp-content/uploads/2019/03/AEB-L-datasheet.pdf
[24] https://www.materials.sandvik/en/products/strip-steel/strip-products/knife-steel/hardening-guide/hardening-programs/sandvik-13c26-batch-hardening/
[25] https://www.materials.sandvik/en/products/strip-steel/strip-products/knife-steel/hardening-guide/hardening-programs/sandvik-13c26-batch-hardening-deep-freezing-70c-95f/
[26] Verhoeven, John D., Alfred H. Pendray, and Howard F. Clark. “Wear tests of steel knife blades.” Wear 265, no. 7-8 (2008): 1093-1099.
[27] https://www.crucible.com/PDFs%5CDataSheets2010%5CdsS35VNrev12010.pdf
[28] https://knifesteelnerds.com/wp-content/uploads/2018/08/Bohler-Uddeholm-CATRA.pdf


I like 1725 and 1990F but i have to tell you… i think it has more to do with oven variation than anything else…
What hardness are you getting now, Andre?
When i first used those temps i got 63.x after 350f temper, but it drifted down -sometimes – to 62-61 – same piece of steel… which seemed not as good as the Rc63 blades, which led me to believe i was overheating some times and got more ra… ithen i reduced to 1700f and 1975f and got “sensible” 62.x after temper, which behaves for lack of a better word predictable… i.e. no unexpected edge failures the just like the Rc63.x blades. Those first ones that were 63, were really good performers, but the risk of the lower values with that heat treat was just not worth it. I still dont know if my oven drifted… i am rebuilding at the moment.
I’m super grateful for your scientific contributions, especially the standardized toughness test data. It’s an improvement over the non-uniform sources I’ve been gleaning comparisons from for years. A quantitative relationship among 8670, 52100, Z-wear, AEB-L, and CPM-154 was previously elusive. I’d still love to see 3V, S35VN, & XHP, and maybe S110V, S690 & Z-Tuff at various HRC.
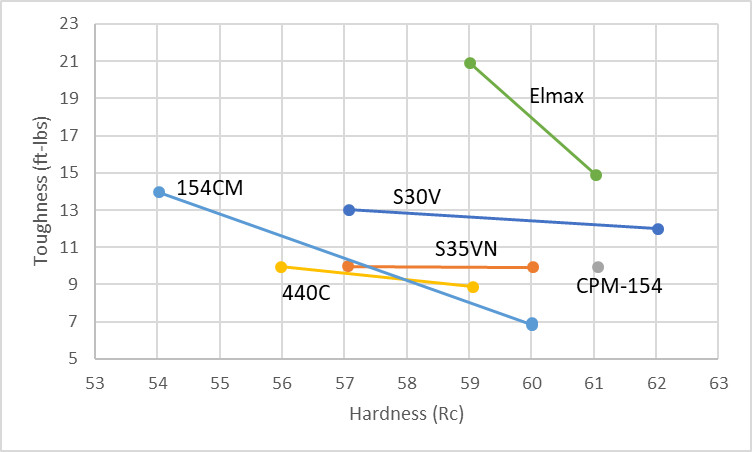
I see one glaring discrepancy between your data and Bohler’s impact testing, which was custom unnotched like yours. They show Elmax as much tougher, not far more brittle, than CPM-154. This was a major surprise for the high alloy composition, and attributable to their 3G PM and perhaps a very specialized HT. I think this certainly bears more investigation at various HRC, because if Bohler’s right, Elmax would be like a stainless Z-wear, i.e. a well-balanced performance leader that could merit near ubiquitous use in more wear resistant applications than AEB-L. Pdf links were removed, though I could send a copy if you want. The data can be viewed in Post #15 https://www.bladeforums.com/threads/so-i-want-to-try-out-elmax.1073013/ By the way, do you use Peter’s HT, your own, or other contributors?
I’m also grateful for the AEB-L HT exploration. It looks like a full hardness medium chopper may be viable, though I’d be interested in toughness estimates and HT schedules for the mid to upper 50’s HRC if anyone knows.
Keep up the good work!
Other steels are being added to the toughness database slowly but surely. Samples of 3V and S35VN are coming among others. Heat treatments have all been performed by knifemakers thus far.
I am familiar with the Bohler-Uddeholm toughness testing, I have the original hosted on my site here: https://knifesteelnerds.com/wp-content/uploads/2018/10/Bohler-Uddeholm-toughness-testing-1.pdf
More samples of Elmax are coming, so if it does better the chart will be updated. I won’t accuse them of manipulating data, but they wouldn’t have shared it if it didn’t show their product in a positive light. Those numbers were generated by Bohler-Uddeholm USA as I understand it.
Either way, Elmax is unlikely to be in the same category of toughness as Z-Wear. Z-Wear has much less carbide volume, and according to Uddeholm’s own numbers (in Sweden), Elmax is much less tough than Vanadis 4 Extra, for example. Elmax is listed as 22J at 59 Rc here: https://www.uddeholm.com/app/uploads/sites/36/2017/08/VANAX-Superclean-eng-1705-e1.pdf while Vanadis 4 Extra is shown as testing at 75J with the same hardness: https://www.uddeholm.com/files/PB_Uddeholm_vanadis_4_extra_english.pdf So looking at their numbers Elmax does not appear to be in the upper echelon for toughness.
If you want upper 50’s for hardness on AEB-L, then use 1925°F and temper at 450-500°F.
Edit: I have re-plotted the Bohler-Uddeholm toughness testing because the original is a little convoluted:
Thanks for the time and experiments with RA, I ve never quenched to room temp. however used freezer in past with the peak hardness at 1065°C that correlates unbelievably well.
Now I use dry ice and seems to be somewhere in between but closer to nitrogen as expected. Great article. Read in one breath curious if your data will confirm my experience from ht aeb-l. Thanks Again.
Hello Larrin,
First 52100, then AEB-L, my two favorite steels. What a treat. 1 What lead you to choose 15 min as soak time? If you ever looked at Sandvik’s technical papers about 12C27, 13C26 and 14C28N, you will see that they recommend about 2 min per mm of thickness soak time and a bit more for above 4 mm (5/32″). For 1/8″~3 mm that’s roughly around 6 min or so. 2 In the same papers and also Roman’s book, it is said that once the lowest temperature is reached, the switch from austenite to martensite is almost instantaneous (at speed of sound I would guess), so why the need or recommendation across the Net for, very often, several hours at very low temp? (my guess was large pieces of the general industry and not thin blades) Any words from you will be appreciated.
Hi Olivier, great questions. Of course I am familiar with the Sandvik and Uddeholm datasheets, I cited them in [23][24][25]. If you look at the Sandvik hardening guide, for example, you will see that there are guides for either belt furnaces (“piece hardening”) or batch furnaces (“batch hardening”). A belt furnace is a continuous process which is used in some industrial settings, they look like this: https://youtu.be/e9PnTPIKd3g?t=77
The speed you set the belt furnace to is important so that the steel is sufficiently soaked at the high temperature. The continuous speed also means that a heat treatment can be dialed in relatively accurately. As you might imagine, the companies would like to set the speed of the conveyor as high as possible to increase throughput.
For “batch hardening” Sandvik recommends 30 minutes at 1975°F as opposed to the shorter hold time in a belt furnace where they recommend 1995°F. A longer time at a shorter temperature makes the steel less sensitive to time/temperature, as the hold time is less critical. I chose 15 minutes as a convenient amount of time that is unlikely to lead to grain growth, while also ensuring that the steel is fully at temperature and that a couple minutes or degrees different is unlikely to greatly affect the final properties. It could be that 10 minutes leads to slightly better properties; I couldn’t say. However, it is unlikely to make a big difference.
Now when it comes to cryo or cold treatment, it is true that the transformation to martensite is near instantaneous. That is why I recommend a 30-60 minute soak in liquid nitrogen, which is just to ensure the entire piece reaches the low temperature. I have a very long 3 part series all about cryogenic processing. I wrote about the claims about longer hold times affecting wear resistance in Cryo Part 3: https://knifesteelnerds.com/2018/12/17/cryogenic-processing-of-steel-part-3/
Great article and interesting steel. Given the high toughness it seems odd that so few makers are using this steel for fixed blades. Any reason for this that you know of?
In the past it has been difficult to buy in bigger thicknesses. And perhaps stainless steels have still had a reputation for poor toughness. My toughness data here hasn’t been available for that long so relative toughness was primarily anecdotal.
Hi, are there any good folding knives made out of aeb-l, or similar steel, that has a fine edge geometry, that you could recommend? Thank you for all your hard work!
I know there are a lot of Kershaw knives in 13C26 and 14C28N. I’m not familiar enough with their product line to know which knives have the thinnest edges.
I just bought a kershaw leek, for $40, saw that it is 14c28n, and was really happily shocked about it, after reading your article. I hope it’s a good knife.
I forgot about the Spyderco Urban sprint run in AEB-L. That might be worth looking into if you like the style.
hi i was looking at njsb new heat treat sheet, they suggest a 15 min pre-heat at 1500f prior to austenizing,what is your opinion on this matter? Straight to ausenizing range or pre-heat?
You can add a preheat step if you want. You can read what it does here: https://knifesteelnerds.com/2018/03/07/austenitizing-part-3-multi-step-austenitizing/
Thanks, I saw your article on the subject after I commented, keep up the good work it’s all really helping!
Some fantastic info thank you 🙏 I am interested in AEB-L but I’m the UK there is VERY limited thickness choices… but I take it when looking at 14c28n we would expect the same toughness as AEB-L on your chart given where it’s been developed from (potentially ever so slightly not quite as tough) given 14c28n potential toughness being based off AEB-L do you expect the manufacturers heat treat to be one to follow (because it was developed for knives?) or would you recommend a slightly tweaked version? Also at what Rockwell would you expect the best toughness/wear resistant trade off to be? Great info as ever and thank you in advance?
I’m waiting on the machining of one set of 14C28N toughness specimens to see how it compares to AEB-L. It heat treated a bit differently than AEB-L for me, you can see in this article: https://knifesteelnerds.com/2019/09/23/nitro-v-its-properties-and-how-to-heat-treat-it/
Thank you for the reply, so 14c28n heat treat..
Austenitizing temp 1900-1950 for 15 mins in steel foil.
Plate quench until you can handle comfortably.
Cryo for 1-2 hour (I find the kiln is below tempering heats at around 2 hours which falls nicely for the cryo time) bring to room temp.
Temper at 320-350 twice for two hours each.
Am I right in thinking this steel is tougher at 61-62 Rockwell as opposed to 59-60 according to the data in the link you attached? You recommend not going above 350 but the Sandvik heat treat is well over that temp to achieve 59 Rockwell which on the face of of it should be tougher than the harder 61-62 achieved by not going above 350 degrees for tempering? I find all this very fascinating and also counterintuitive although I maybe interpreting your data (and Sandvik) incorrectly 🤦♂️ As always LarrinMarch thank you for digging deep in your tests for us mortals 👍
No I’m not recommending the same heat treatment as nitro-v. There is a chart for austenitizing temperature vs hardness to see how 14C28N heat treats differently. I don’t have enough information to recommend an exact heat treatment. It may require higher temperature like 2000F.
Hey larrin I know this post is like a year old but I have a couple of questions.
1.) Is 14c28n similar to nitro-v? Basically aebl with added nitrogen? What are your thoughts on it?
2) this may be a dumb question. But if you can achieve desired hardness post quench why temper? Is it more for stress relief after heat treat and cryo?
Thanks for all you do. I still think you should develop your own steel some day!
14C28N has better corrosion resistance than Nitro-V. The CATRA testing also found a it better result for 14C28N. They also heat treat differently.
Tempering improves toughness.
For those of you wondering about dry ice cryo treatment. I tested two conditions and got similar results to liquid nitrogen. I cryo-treated my steel for 30 minutes in a bath of ethanol and dry ice. For the 1750F prequench, austenitize at 1975F, 30 minutes of dry ice cryo, and 350F temper I got a hardness of 62.6 HRC. For no pre-quench, austenitize at 1975F, 30 minutes of dry ice cryo, and 350F temper I got a hardness of 61.7 HRC.
My steel was from Alpha Knife Supply.
I’m having a hard time finding aebl 11 ga. Can anyone help me ? Thanks
https://www.alphaknifesupply.com/shop/aeb-l-stainless-steel
Is there an angle or angle range which would be best suited for sharpening this steel for a larger fixed blade knife? Would 11 degrees make the most of this steels attributes? Would widening the angle lose some of the edge retention qualities for this steel?
If you’re making a large blade you probably want something around 20 dps to be able to withstand chopping.
Hey what hardness would you recommend for chef knives? I prefer Japanese style gyuto knives so much more than German style chef knives. I am planning to get a custom aeb-l for everyday work knife would 64 hrc still be good choice for me? or maybe 63? I the knife is planned to be 230g and blade length at 240mm and heel to spine at 60mm.
Sure 64 Rc should work fine. Let us know how it goes.
Hi Larrin, absolutely loving using AEBL right now and as you say it seems to be the Goldilocks steel but one thing I seem to of missed is this…. would you recommend putting in the
AEB-L at the start when the kiln is cool or waiting until it gets to temperature before putting it in? Thanks in advance
https://knifesteelnerds.com/2021/04/13/how-even-is-an-evenheat-how-to-operate-furnaces-effectively/
Ok I see 👌 I also see I need a decent thermometer for the kiln for when I’m tempering now lol
I’ll start adding these in when I’ve equalised the kiln at the aust temp, thanks for your time Larrin you’ve got almost too much info now for a layman like me on your site 🙂🇬🇧
Hi Larrin, are modern razor blades still made of AEB-L /13C26 and what hardness are they ? Thanks.
Yes though I couldn’t give you a percentage or anything. It’s been a while since I looked up hardness values on razors, Dr. Verhoeven lists them as 62-63 Rc in this article: https://knifesteelnerds.com/2021/01/11/what-causes-razor-blades-to-dull/
Do you know if any other steel is used for razor blades these days ? Also I wonder why major kitchen knives manufacturers ( i.e. Zwilling, Wuesthof) don’t use AEB-L for their knives since it’s not expensive and can be easily processed but can bring an advantage over widely used X50CrMoV15.
zwilling has alot of knives made from aeb-l but they renamed it as fc61 fine carbide 61 hrc. you can find them in kramer miyabi and even on some of their japanese made zwilling knives.
I know it is kind of old, but has anyone tried to heat treat AUS-6 to AEB-L’s high hardness that you know of? AUS-6’s composition is quite similar to AEB-L except I’m wondering if the higher Mn & Silicon in AUS-6 with the added .50% Nickel would prevent the high hardness achievable with the AEB-L?
I was also wondering if the Japanese make their own razors from it rather than importing Sandvik steels? I know that some knife suppliers sell 6A knife kits and it is reported as pretty hard, though no hardness numbers are released.
I haven’t seen any high hardness AUS-6. Aichi doesn’t list razors anywhere on their site so perhaps they indicates they aren’t selling it for them but it couldn’t be ruled out. Hitachi does list razors as an application for GIN-5, however.
Great resource Larrin, very much appreciated!
Do you have any recommendations for forging AEB-L, and how to treat it after forging?
Thanks!
The most common mistake when forging stainless is heating it too hot. About 2100F is good. Then anneal it, such as by using a cycle from the 154CM datasheet: http://www.crucible.com/PDFs/DataSheets2010/ds154cmv12010.pdf
After that austenitize, quench, etc like normal.
Dear Larrin,
The chart Hardness vs weight % carbon with data from different authors seems to be taken from an article in scientific litterature. Can you share the reference please (I did not find any in your post). I am wondering if the carbon percent (X-axis) is for general carbon content or carbon in solid solution.
Thanks
It was cited in this article about martensite hardness: https://knifesteelnerds.com/2018/04/10/what-makes-quenched-steel-so-hard/
Dear Larrin,
Did you ever by chance compare the edge retention and/or toughness of cryogenically treated AEB-L against room temperature or freezer cooled AEB-L, tempered to the same hrc?
I have done a toughness comparison but it hasn’t been published yet.
Dear Larrin
I tried to heat treat aeb-l according to your article a few times but I didn’t get the results I think I should and my kitchen knife doesn’t have the edge retention it should (i can tell a clear difference after a few weeks of use). Should I use foil to prevent decarb? Do you know how fast the steel should cool down (like you can read from a cct diagram). I use aluminum plates but the are ¾ *2*6 inch, could it be that my plates are too small to cool the steel down fast enough?
Thanks
You need to use foil to prevent decarb.
I’m making a small slicer. .120″ thick, 8″ overall length. 1-1/4″ widest point.
I have a kiln and antiscale. Two 1″ thick x 3″ x 12″ aluminum bars.
I plan on going w 1925 15 minutes then the plate quench then my deep freezer.
I thought I read letting it get to room temp before the freezer gains nothing. Dry ice and LN are not really an option.
Couple questions if you please.
How cool do I plate quench it before I put it in the freezer? I know you have a specific time to get it down to a certain temp to harden it but I couldn’t find what that time and temp is for aeb-l.
How fast will the plates cool it?
Thanks.
How fast it is cooled down will depend on the size of the knife, whether you hit it with compressed air at the same time, etc. You can check with a laser thermometer or something if you want to get a feel for how fast it is cooling. Should take less than 30 seconds.
Very surprised by the performance of this steel that’s been under my nose this whole time!
A shame about the difficulty in forging- its a bit of a pipe dream but we could make some pretty wild choppers with it 🙂
Outstanding article. Thank you.